HPV Profiler
Next-Generation Sequencing for Advanced HPV Genotyping and Gene Expression
Product Overview:
Background information
The HPV Profiler employs targeted next-generation sequencing (NGS) technology to provide comprehensive genotyping and/or gene-expression analysis for 20 high-risk or potentially high-risk Human Papillomaviruses (HPV). When used on DNA samples, this assay delivers accurate detection and differentiation of these genotypes for up to 48 samples in a single run. When run on cDNA samples, it additionally quantifies transcriptional activity of key viral oncogenes (E2, E6, E7 and the E6* splice variant), offering insights into which infections are potentially clinically relevant. Designed for researchers, the HPV Profiler provides a deeper understanding of HPV biology.
Why choose HPV Profiler?
In contrast to traditional HPV tests, HPV Profiler quantifies transcriptional activity, while giving sequence information that allows genotype annotation with unparalleled resolution, even at strain level. This provides a clear signal of clinically relevant infections. Moreover, it delivers this readout across 20 high- and low-risk subtypes, as opposed to clinically insignificant (transient) HPV infections that are detected by conventional tests. As vaccination campaigns are expected to shift HPV genotype distributions, this becomes critical for accurate monitoring. Moreover, HPV Profiler integrates seamlessly with standard DNA purification or first-strand cDNA synthesis workflows. Also, due to its targeted nature, HPV Profiler requires minimal sequencing capacity per sample, reducing sequencing cost per sample dramatically while retaining a high sequencing depth. The result is a fast, cost-effective NGS solution which does not require expert knowledge due to our automated Cirnalyzer data processing and analysis software, delivering clear, publication-quality results
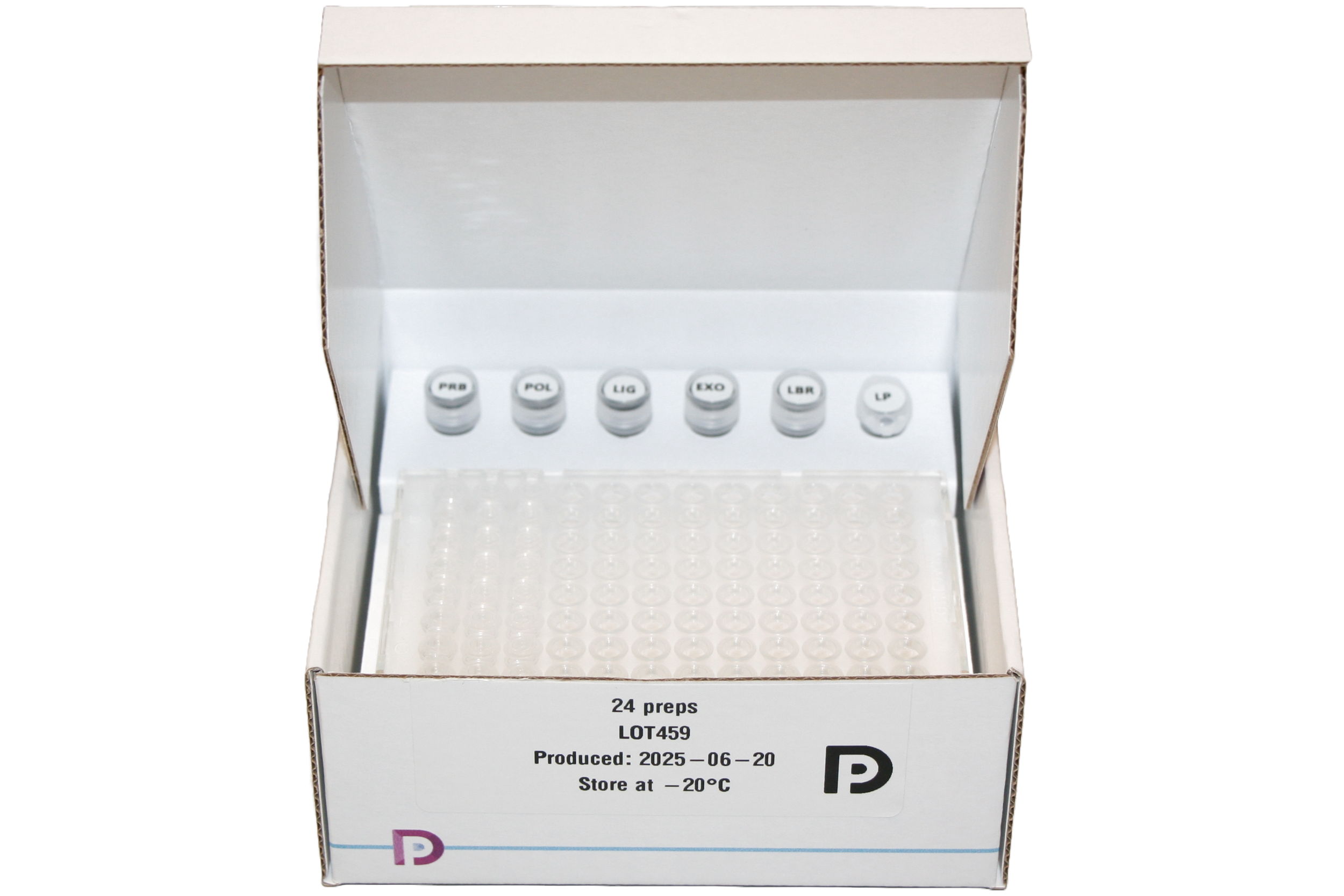
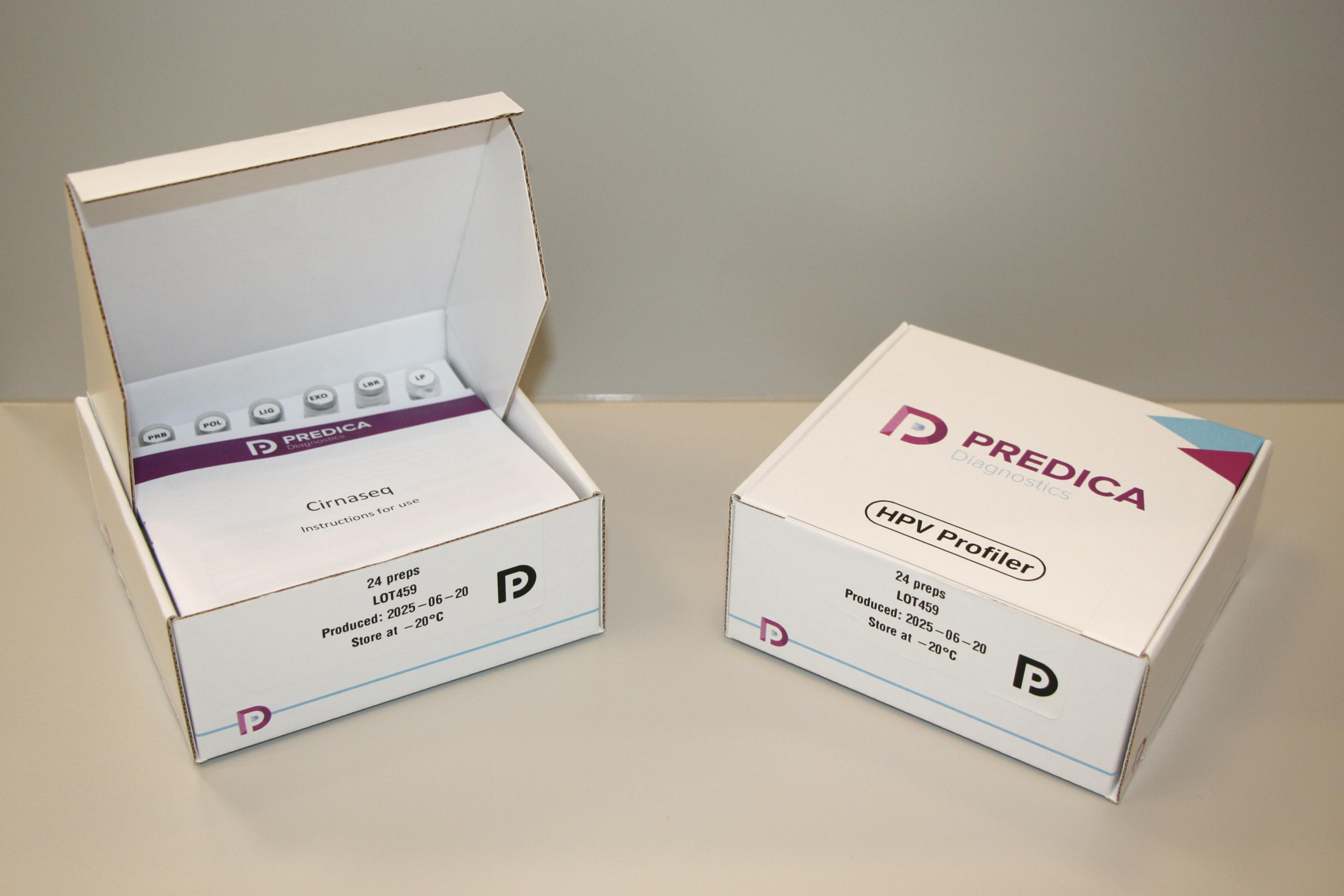
Kit components
Each kit includes assay reagents, a barcoded primer plate, and an empty pre-labelled tube for the return of your library. Note: AMPure XP Reagent is not included in the kit. Sequencing is provided by Predica Diagnostics at no additional charge.
Documents & Downloads:
Flyers:
Datasheets:
For Research Use Only. Not for use in diagnostic procedures.
Contact:
Interested in the HPV Profiler?
Complete the form below to receive the official Instructions for Use (IFU) and our team will be in touch to answer your questions.